The relief of postoperative pain continues to pose a pri- mary therapeutic challenge for clinicians. Despite the development and implementation of novel analgesic strate- gies over the past several decades, more than 50% of patients experience moderate-to-severe pain, even after “minor” surgical procedures.1–3 Traditionally, shorter-acting opioids like morphine or hydromorphone have been administered as intermittent intravenous boluses to provide postopera- tive analgesia. However, this approach can produce widely fluctuating blood opioid concentrations, resulting in clini- cal responses that can range from inadequate pain relief to profound sedation and respiratory depression. Postoperative pain may be more effectively managed with patient-con- trolled analgesia devices, but this approach requires complex programed infusion systems, patient cooperation and edu- cation, and can also result in significant variability in drug concentrations (a bolus is administered when the patient experiences pain).The use of regional anesthetic techniques can provide high-quality analgesia but is not possible in all patients and may not provide complete pain relief.
Methadone is an alternative opioid with a long half- life that provides stable blood concentrations after a sin- gle intraoperative dose, without the fluctuations associated with repeated injections of high clearance agents like mor- phine or hydromorphone. It is a potent μ-receptor ago- nist with the longest elimination half-life of the clinically used opioids.4 Due to its high oral bioavailablity and long duration of clinical effect, methadone is used (along with buprenorphine) for medication-assisted treatment of opi- oid abuse disorder (oral methadone maintenance replacing intravenous diamorphine [heroin]).The efficacy and safety of methadone has been extensively studied in this setting.5 However, there have been relatively few clinical investiga- tions examining the impact of intraoperative methadone use on clinical outcomes.
Methadone is an opioid that possesses several unique properties that may be advantageous in patients undergoing surgical procedures. It has a long elimination half-life of 24 to 36 h.6,7 When dosing methadone intraoperatively, the goal is to target blood concentrations in excess of the minimal
Clinical Trials examining the effect of intraoperative Methadone on Postoperative outcomes
In 1982, Gourlay et al.6 published a small study describ- ing the effect of a single dose of methadone, administered at induction of anesthesia, on postoperative pain scores and analgesic requirements. Since this initial publication,
This article has been selected for the Anesthesiology CME Program. Learning objectives and disclosure and ordering information can be found in the CME section at the front of this issue. This article is featured in “This Month in Anesthesiology,” page 1A. For a downloadable PPT slide containing this article’s citation information, please visit https:// anesthesiology.pubs.asahq.org/ss/downloadable_slide.aspx.
Submitted for publication October 5, 2018. Accepted for publication March 13, 2019. From the Department of Anesthesiology, NorthShore University HealthSystem, University of Chicago Pritzker School of Medicine, Evanston, Illinois.
Copyright © 2019, the American Society of Anesthesiologists, Inc. All Rights Reserved. Anesthesiology 2019; 131:678–92. DOI: 10.1097/ALN.0000000000002755
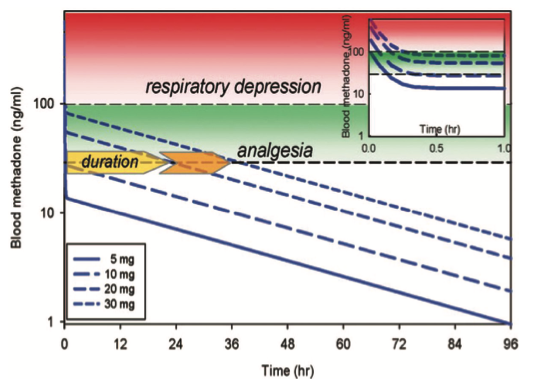
Fig. 1. relationship between methadone dose and duration of effect. Simulated methadone blood concentrations versus time based on pharmacokinetic parameters, the minimal effective analgesic concentration (approximately 30 ng/ml), and the threshold for significant respi- ratory depression (approximately 100 ng/ml), as determined by Gourlay et al.,6,7 Data are shown for bolus methadone doses of 5, 10, 20, and 30 mg, with the estimated duration of analgesia of less than 0.5, less than 0.5, approximately 24, and approximately 36 h, respectively. the inset shows plasma concentrations for the first hour after dosing. because of rapid redistribution, anticipated respiratory depression would be less than 30 to 45 min, even at the higher single bolus doses. reprinted with permission from e. D. Kharasch.4
investigations in a variety of patient populations have exam- ined the effect of methadone, administered in the oper- ating room (or operating room and postanesthesia care unit [PACU]), on postoperative recovery. In these clinical trials, analgesic requirements or pain scores were the pri- mary outcome measures (all pain scores were reported on an 11-point verbal analog scale with 0 = no pain and 10 = worst pain imaginable). Despite the use of similar proto- cols designed to treat postoperative pain in the methadone and control groups, most investigations demonstrated that patients administered methadone had lower pain scores and postoperative narcotic requirements, which was likely due to the prolonged analgesic effects produced by methadone.
Methadone in patients Undergoing major Inpatient Surgery
In the first perioperative investigation examining the phar- macokinetics and pharmacodynamics of intraoperative methadone, Gourlay et al.6 administered 23 subjects (11 spi- nal fusion patients and 12 general surgical patients) 20mg of methadone at anesthetic induction. After surgery, 9 subjects (39%) required no postoperative pain medication, 6 subjects (26%) requested nonopioid analgesics (first supplemental dose at 27 h), and 8 subjects required opioid medication (first supplemental dose at 18 h). In a subsequent study, 16 patients (14 undergoing general surgical procedures and 2 under- going orthopedic surgery) were given 20mg of methadone
at anesthetic induction, with supplemental methadone pro- vided in the PACU until the patients reported no pain.7 In contrast to their earlier study, all of the subjects required additional methadone in the PACU (median dose 10mg). However, once patients were comfortable, the mean dura- tion of analgesia was 21 h, and mean pain scores were 1.5 on a 0 to 10 scale.The minimum effective blood concentra- tion of methadone was determined to be 57.9 ± 15.2ng/ ml, which was higher than that determined in their ear- lier investigation (31.6 ± 11.1ng/ml),6 suggesting that the minimal effective concentration may vary in relation to surgical procedure.The same investigators then performed a randomized, double-blinded trial in which 20 patients undergoing upper abdominal procedures were administered either methadone or morphine.15 Twenty milligrams of either agent were given at anesthetic induction, with 5-mg boluses provided in the PACU and surgical wards until patients were comfortable. Both cohorts required 8 to 9 mg in the PACU to achieve initial pain control. However, the time from initial pain control until the first supplemental dose of opioid needed was significantly longer in the meth- adone group (21 h) compared to the morphine group (6 h), and total requirements for opioids were lower in the patients given methadone (12 mg vs. 41 mg in the morphine group).
In another early clinical trial (1983),24 patients undergo- ing elective total hip replacement surgery were randomized to receive 10mg of methadone at induction of anesthesia
or at the end of the procedure.16 On postoperative day 1, the requirements for opioid pain medication were approx- imately two-fold higher in the group given methadone at the end of surgery, suggesting that dosing before the proce- dure is beneficial.
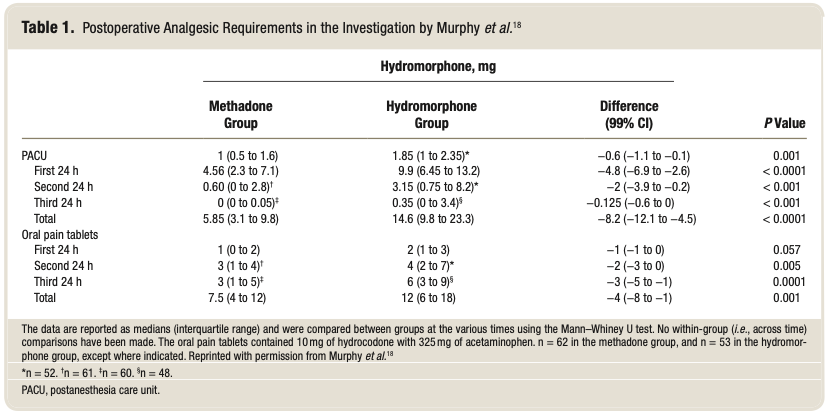
Two investigations examined the use of methadone in adults undergoing major spine surgery. Gottschalk et al.17 ran- domized 29 patients to receive either 0.2 mg/kg of methadone at induction or a continuous sufentanil infusion throughout surgery. Forty-eight hours after surgery, opioid requirements and pain scores were approximately 50% lower in the group administered methadone. In a larger investigation enrolling 120 patients, the subjects were randomized to be adminis- tered methadone 0.2mg/kg at the start of surgery or hydro- morphone 2mg at the end of surgery.18 In the methadone group, opioid requirements were reduced by more than 50%, pain scores were less at 21 of the 27 assessments, and over- all satisfaction with pain management was higher, during the first 3 postoperative days when compared to patients given hydromorphone (table 1).Two further methadone studies in pediatric spine patients have been performed (discussed in the Methadone in Pediatric Surgical Patients section).19,20
Intraoperative methadone has also been examined in gynecologic and obstetric patients. In a randomized, dou- ble-blinded investigation in hysterectomy patients, Chui and Gin21 administered either 0.25 mg/kg of methadone or morphine at anesthetic induction, with further increments given in the PACU if analgesia was required.The mean total doses of methadone (0.43 mg/kg) and morphine (0.45 mg/ kg) required did not differ between groups. However, 10 of the 15 patients given methadone required no further post- operative morphine, and pain scores on a 0 to 10 scale were lower for the first 48h in this group (1 to 2 vs.3 to 5 in the morphine group). In another single-blinded investigation in
women undergoing hysterectomies, 40 patients were ran- domized to receive either 20mg of morphine or metha- done at induction of anesthesia, with the same drug given for pain in the PACU and surgical wards for analgesia.22 Patients in the methadone cohort required less opioid in the PACU (2.0 mg vs. 4.4 mg) and on the wards (4.5 mg vs. 42.3 mg), and pain scores on a 0 to 10 scale were less in this group (1.9 vs. 3.4), compared to the morphine cohort, over the 72-h study period.A further retrospective case-control investigation examining outcomes in elective or emergent caesarian deliveries compared 25 patients administered methadone (mean dose of 0.17mg/kg) to 50 control sub- jects receiving fentanyl, morphine, or both.23 Patients in the methadone cohort reported lower pain scores and required 40% less opioids in the first 48 postoperative h.
The analgesic effects of methadone have also been examined in cardiac surgical patients. Two studies from Brazil compared recovery from cardiac surgery in patients randomized to receive either methadone or morphine. In a double-blinded investigation, Udelsmann et al.24 admin- istered patients 20mg of methadone, 20mg of morphine, or saline (control) after induction. Compared to the other two groups, patients administered methadone required sig- nificantly less postoperative analgesics (45% needed none), and pain scores on a 0 to 10 scale were less (0.5 vs. 2.8 in the control group) in the first 24 postoperative h. Carvalho et al.25 randomized 100 patients to be given 0.1mg/kg of methadone or morphine at the end of cardiac surgery. Significantly fewer patients required postoperative opioids in the methadone group (29% vs. 43% in the morphine cohort), and pain scores on a 0 to 10 scale were reduced in this group at 24 h (1.9 vs. 2.9 morphine cohort).
In the largest intraoperative clinical trial using meth- adone, Murphy et al.26 randomized 156 cardiac surgical
patients to be given either 0.3 mg/kg methadone or 12 μg/ kg fentanyl before cardiopulmonary bypass. Postoperative opioid requirements and pain scores were reduced by approximately 40% during the first 3 postoperative days in the methadone group, and patient satisfaction with pain management on a 100-mm verbal analog scale was higher in these subjects (90 to 100 vs. 70 to 90 in the fentanyl group). These findings demonstrated that despite a long time period between anesthetic induction and tracheal extubation in the intensive care unit, a dose of methadone given before surgery provided a prolonged analgesia benefit (Porter et al.16 observed that patients administered metha- done at induction of anesthesia had postoperative opioid requirements that were approximately 50% less than those given methadone at the end of surgery).
Lower-dose methadone in Ambulatory Surgical patients
A smaller dose of methadone may be effective in producing long-lasting analgesia in patients undergoing ambulatory surgical procedures. Simoni et al.27 randomized 126 patients undergoing laparoscopic surgery to receive either 0.1mg/ kg methadone, 2 μg/kg clonidine, or normal saline (con- trol) before the procedure. A total intravenous anesthetic technique with remifentanil (which may promote acute tolerance and hyperalgesia)28 was used in all subjects. The number of patients with pain in the immediate postopera- tive period was significantly lower in the methadone group compared to the clonidine and control groups (approxi- mately 50% less).
The efficacy and safety of methadone in ambulatory surgical patients (most undergoing laparoscopic cholecys- tectomy, tubal ligation, salpingectomy, oophorectomy, or salpingectomy with oophorectomy) was assessed in a ran- domized, double-blinded, dose-finding study.29 At induc- tion of anesthesia, 40 patients were administered methadone (initially 0.1mg/kg ideal body weight and then 0.15mg/ kg ideal body weight), and 20 patients were given standard shorter-acting opioids (controls). Opioid consumption, pain intensity, and opioid side effects were assessed in the hospital and for 30 days postoperatively using home diaries. In-hospital nonmethadone opioid use (morphine equiv- alents) was less in patients given 0.1 and 0.15mg/kg of methadone (7.1 and 3.3 mg, respectively) compared to the control group (35.3 mg, P < 0.001). In the first 30 postop- erative days, patients administered 0.15mg/kg methadone reported less pain at rest (P = 0.02) and used fewer opioid pills than controls (5 vs. 10, P < 0.0001).
Methadone in pediatric Surgical patients
The pharmacokinetics of methadone in adolescents under- going major spine surgery was examined in two investiga- tions. In the first, 31 children (ages 5 to 18 yr) received a dose of 0.1, 0.2, or 0.3 mg/kg of methadone at anesthetic induction.19 This cohort was compared to a similar group
not administered methadone. Methadone pharmacokinet- ics were linear over the dose range studied (fig. 2).Although analgesic requirements were reduced with increasing doses of methadone, the differences were not statistically signifi- cant; however, the study was likely underpowered to detect differences in this secondary endpoint. A similar study was performed in 17 adolescents (ages 12 to 19 yr) given 0.25mg/kg of methadone before surgical incision.20 The authors observed that the mean methadone concentration was less than 58 μg·L-1 by the first hour after administration (a previous investigation established the minimum effective blood concentration for analgesia was 58 μg/l for more pain- ful operations);7 the authors recommended that additional methadone should be administered to ensure adequate plasma concentrations for 24h. Pain scores and analgesic requirements were not reported in this investigation.
A double-blinded study in 35 children (ages, 3-7 yr) undergoing major surgical procedures randomized subjects to either 0.2mg/kg of methadone or morphine at induc- tion, with supplemental doses of the same agent provided for analgesia in the PACU.30 During the first 3 postoperative days, fewer patients in the methadone group had severe pain scores (18%) compared to the morphine group (35%), and analgesic requirements were less in the methadone cohort. An addition retrospective investigation examined four types of anesthesia for pediatric patients undergoing the Nuss procedure for correction of pectus excavatum: gen- eral anesthesia with standard short-acting opioids, epidural with general anesthesia, multimodal anesthesia (ketamine, dexmedetomidine, and clonidine patch), and multimodal
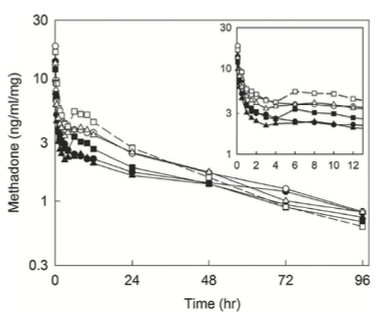
Fig. 2. Dose-adjusted plasma concentrations of methadone after intravenous administration.19 Subjects received 0.1 (circles), 0.2 (squares), or 0.3 (triangles) mg/kg of racemic (R,S)-methadone hydrochloride. Solid symbols and lines show R-methadone, and open symbols and dotted lines show S-methadone. each data point is the mean. the inset shows the period from 0 to 12 h. reprinted with permission from Sharma et al.19
anesthesia with methadone (0.1 mg/kg).31 Compared to the other three groups, patients in the multimodal anesthesia cohort with methadone had the lowest total postoperative opioid use (50% less than the epidural group), the least time with uncontrolled pain, and the shortest hospital length of stay.
Important limitations of Published Clinical Trials
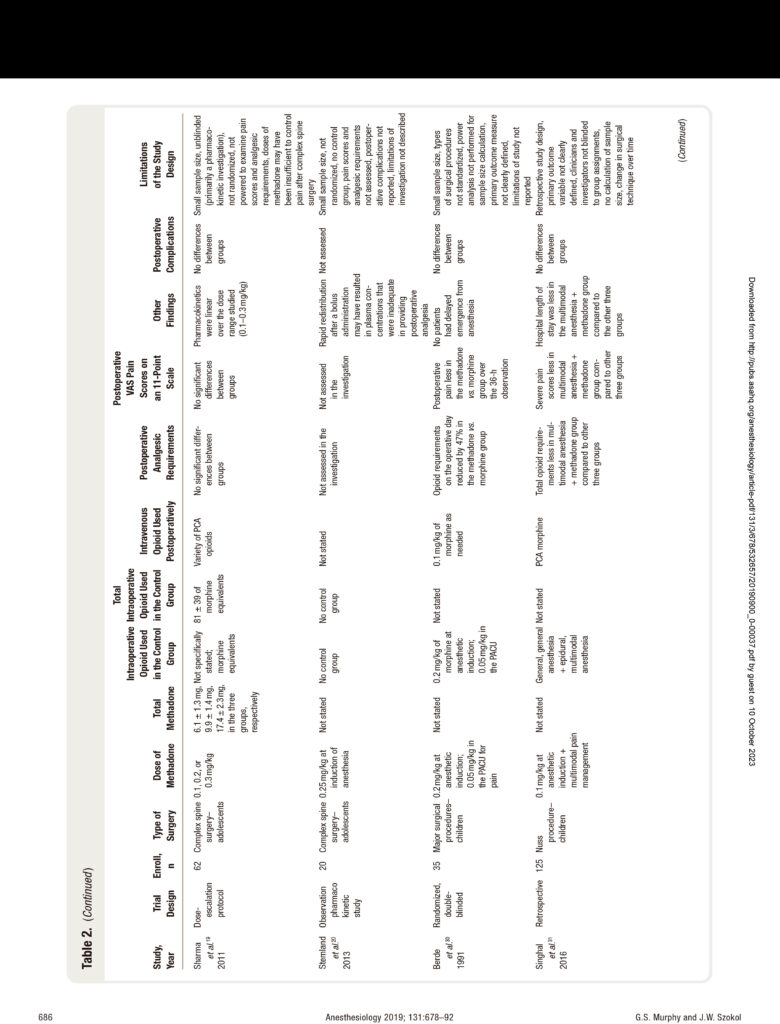
Studies examining the use of methadone in the perioper- ative setting have documented that a single dose adminis- tered intraoperatively produces a prolonged analgesic effect that can persist during the period of the most intense post- operative pain (postoperative days 1 through 3). Despite this encouraging research, there are limitations to many of the clinical trials. Most importantly, the majority of prospective clinical studies enrolled only a small number of patients. Only 4 investigations enrolled 100 patients or more, and 11 of the remaining 13 investigations examined less than 50 subjects. Such small sample sizes can produce false pos- itive results or overestimate the magnitude of an associa- tion. Furthermore, only a few investigations examined the potential analgesic benefits of methadone in conjunction with other opioid-sparing agents.At the present time, there is a need for larger-scale, double-blinded investigations to define the efficacy and safety of intraoperative methadone. The limitations of the published research are presented in table 2.
Safety of intraoperative Methadone
A primary concern related to the use of long-acting opi- oids is the potential for prolonged respiratory depression. In randomized trials, no differences in the incidence of respiratory depression (respiratory rates less than 8 to 12 breaths/min) or hypoxemic events (oxygen saturations less than 92 to 90%) were observed between methadone and control groups during the PACU admission, on the sur- gical wards, or in the intensive care unit.15,18,19,21,22,24–26,29,30 Similarly, no episodes of adverse respiratory events were reported in patients administered intraoperative methadone in observational or retrospective investigations.6,7,16,20,23,31 No patients given methadone required naloxone infusions for prolonged respiratory depression.
Clinical trials suggest that methadone does not appear to increase the risk of other opioid-related side effects. Studies that have assessed patients for level of postopera- tive sedation have documented that no differences existed between subjects administered intraoperative methadone and those given conventional opioids.18,19,21,22,26,29,30 The observed incidences of postoperative nausea and vomiting did not differ between the methadone and control groups, with the exception of a higher risk in methadone patients in the PACU (but not on the wards) noted by Chui et al.21 and a lower risk in methadone patients in the intensive care unit observed by Udelsmann et al.24 No adverse cardiac
events related intraoperative methadone administration have been described in the published literature. Only one investigation examined the effect of methadone on bowel function (no differences were observed between the meth- adone and hydromorphone groups in the time to first fla- tus or bowel movement after spine surgery).18 However, it is important to note that the majority of these clinical trials were small and not powered to assess safety outcome mea- sures, particularly rare events such as significant respiratory depression. In addition, high-risk patients were excluded from enrollment in many studies. Although limited data from randomized studies suggest that the risks of metha- done do not exceed conventional shorter-acting opioids, additional information from larger-scale investigations is needed (particularly related to respiratory depression).
One case report from 1976 describes an 81-yr-old female with normal renal and hepatic function who received 30mg of methadone (0.7mg/kg) for a mitral valve pro- cedure.32 The patient was extubated after receiving 1.0mg of naloxone and subsequently required additional naloxone every 2 to 4h for the next 8 days.The prolonged effect was likely related to the large dose given and the patient’s advanced age.
Dunn et al.33 published a retrospective review of periop- erative adverse events in patients administered intraoperative methadone (mean dose, 11.5mg) for major spine surgical procedures.The records of 1,478 patients undergoing these operations over a 5-yr period were examined. Respiratory depression (fewer than 8 breaths/min) was observed in 37% of patients, and hypoxemia (oxygen saturation less than 90% or the need for more than 2 l of nasal cannula oxygen flow to maintain oxygen saturation at greater than 96%) was noted in 80% of patients. Nalaxone was needed in 2% of patients, and 1.5% required reintubation. Although a high incidence of adverse events was described in this investi- gation, an important limitation is that the study did not include a propensity-matched control group given shorter- acting opioids.
Several synthetic opioids, such as methadone, inhibit the serotonin transporter at clinically relevant concentra- tion, resulting in increases in intrasynaptic levels of sero- tonin.34 Case reports have described the development of serotonin syndrome in patients on chronic methadone maintenance therapy administered other serotonergic medications including monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, serotonin–nor- epinephrine reuptake inhibitors, and tricyclic antide- pressants.34 Serotonin syndrome has not been reported in patients administered intravenous methadone periopera- tively. However, serotonin syndrome should be suspected if a patient on antidepressants given methadone develops altered mental status, autonomic instability (fever, tachy- cardia), or neuromuscular abnormalities (rigidity, tremor, clonus) in the perioperative period.


Pharmacogenomics of Methadone Metabolism
There may be considerable interindividual and intraindi- vidual variability in the disposition of methadone, which may be related to genetic polymorphism of hepatic cyto- chrome P450 genes. Methadone is cleared primarily by P450 (CYP)-catalyzed N-demethylation to inactive 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine and a small amount of urinary excretion of the unchanged drug.35 The P4502B6 (CYP2B6) genotype affects plasma metabolism and clearance, with certain allele carriers (CYP2B6*6) having higher methadone concentrations and slower elim- ination, whereas other carriers (CYP2B6*4) have faster elimination and lower plasma concentrations.35 However, this effect is significantly greater after oral methadone administration compared to intravenous administration (which may explain the unpredictability of methadone dosing when initiating oral therapy). In addition, medica- tions that may induce (phenobarbital, phenytoin) or inhibit (fluoxetine, sertraline, ticlopidine) CYP2B6 may potentially influence plasma concentrations of methadone.
Questions to Be addressed in Future Research
What Is the Optimal Dose of methadone to be Administered to patients Undergoing Various Surgical procedures?
The dose of methadone that will result in prolonged anal- gesia without inducing respiratory depression has not been clearly defined in the literature. Furthermore, the minimal effective concentration of methadone required for pain relief may vary dependent upon the surgical procedure.6,7 In the initial investigation by Gourlay et al.,6 20mg was administered at induction, with subsequent studies by this group administering an additional dose of 8 to 10mg in the PACU to achieve sustained analgesia.7,15 A variety of doses have been used in clinical trials, ranging from 0.1 to 0.3 mg/kg, with the majority of studies using a dose of either 0.2mg/kg or a fixed dose of 20mg.
With the exception of the investigation by Sharma et al.,19 dose–response studies of methadone in the periopera- tive period have not been performed. It is likely that more painful operations (major spine surgery) require larger doses of methadone, and the administration of 0.25 to 0.3 mg/ kg may be insufficient.19,20 In the pharmacokinetic study by Stemland et al.,20 few patients maintained steady-state plasma levels above those recommended in Gourlay et al., for painful procedures (58 ng/ml). Simulation modeling in this investigation suggested that a significantly higher dose of intraoperative methadone (a second bolus of 0.35 mg/kg of methadone at 4 h) would be required to achieve sustained plasma concentrations for 24h (or alternatively provid- ing subsequent dosing based upon pain severity [0.03mg/ kg for mild pain and 0.05mg/kg for severe pain every 4 h]). In contrast, other studies have documented a signifi- cant opioid-sparing effect from doses as low as 0.1mg/kg
of methadone in patients undergoing major surgical pro- cedures25,31 (although the analgesic benefit of this lower dose was relatively modest in the investigation by Carvalho et al.,25 and the study by Singhal et al.31 used methadone 0.1mg/kg as a component of a multimodal pain manage- ment strategy). In laparoscopic surgical patients, doses of 0.1 to 0.15 mg/kg ideal body weight may provide sufficient analgesia with no side effects (median dose of 9mg in the higher-dose group).29
Only three studies reported whether methadone was dosed on actual or ideal body weight.18,25,29 Interpatient variability in dosing may be minimized if ideal body weight is used, yet may result in minimal effective blood concen- trations below the threshold for analgesia in some patients. In contrast, dosing on actual body weight in obese patients may result in high blood concentrations of methadone that result in prolonged respiratory depression. The adminis- tration of methadone may be simplified by giving a stan- dard dose at induction (10 or 20 mg), dependent upon the expected degree of postoperative pain.
Determining appropriate dosing of methadone may be further complicated in the opioid-tolerant patient. Patients presenting for certain surgical procedures (complex spine surgery) are often prescribed potent opioids for preexist- ing neuropathic pain.These patients will likely benefit from larger doses of intraoperative methadone; however, appro- priate dosing is dependent upon the degree of tolerance that is present at the time of surgery.Titration of additional methadone in the PACU, based upon degree of sedation and respiratory rate, may be of particular benefit in this patient population.7,15
Is methadone Safe in High-risk patient populations?
With the exception of studies performed in cardiac surgical patients, clinical trials examining perioperative methadone use have enrolled relatively healthy patients without signif- icant medical comorbidities.The safety of methadone in a higher-risk patient population (the elderly, those who are morbidly obese, or those with cardiovascular disease) has not been documented in the published literature. Gouley et al.6 observed that the methadone terminal half-life was positively correlated with patient age, which suggest that more careful dosing of methadone is required in the elderly.
The efficacy and safety of methadone has not been spe- cifically assessed in morbidly obese patients, although these patients were not excluded from many clinical trials. Obese patients, particularly those with obstructive sleep apnea, may have a greater sensitivity to the respiratory depressant effects of opioids, although high-quality evidence support- ing this belief is lacking.36 More cautious dosing and mon- itoring of the effects of methadone may be required in this patient population. It is possible, however, that by reducing the number of supplemental doses of opioids used postop- eratively, intraoperative methadone may attenuate the risk of hypoventilation and hypoxemia after surgery. Further
studies are required to define optimal dosing practices in this patient population.
Is Intraoperative methadone Associated with an Increased risk of Qt prolongation and Cardiac Arrhythmias?
Patients receiving methadone maintenance therapy for opioid dependence disorder have an increased risk of QT prolongation, torsade de pointes, and cardiac death.37 The potential for QT prolongation and the development of arrhythmias appears to be directly related to dose and chro- nicity of use in this patient population.37 The effect of a single intravenous dose of methadone on the QT interval and risk of arrhythmias has not been specifically defined in a randomized trial. However, a higher incidence of adverse cardiac events has not been observed in patients adminis- tered perioperative methadone in clinical studies, and a systematic review of case reports of torsade de pointes did not describe this event after intraoperative methadone use.38 However, conclusions relating to cardiac safety are limited by the small size of the majority of clinical trials.
In an independent ancillary study to the VINO trial, Nagele et al.39 examined surgical and anesthetic factors (drugs, stress, hypothermia, electrolyte disturbances) asso- ciated with QTc (QT interval corrected for heart rate) prolongation in 469 patients undergoing major noncardiac surgery. In the PACU, a QTc interval of 440ms or more was observed in 51% of patients, which resolved in all sub- jects by the first postoperative day. A number of medica- tions used in the perioperative period were associated with QTc prolongation, including methadone (mean change in QTc interval of 30.7 ms). In a retrospective analysis of 1,478 patients given methadone (in addition to other periopera- tive medications affecting the QT interval) for major spine surgery, 58.8% of patients demonstrated QTc prolongation on a postoperative electrocardiogram (defined as more than 440 ms for men or more than 460 ms for women).33 Torsade de pointes was not observed in any patients.
Does methadone Use in the Operating room reduce the risk of Development of Chronic postsurgical pain?
Acute pain after surgery is a primary risk factor for the development of chronic postsurgical pain, which is observed in 10 to 50% of patients.40 Furthermore, pain triggers NMDA receptor activation, resulting in prolonged increases in nociceptive transmission.This process has been postulated to contribute to hyperalgesia and allodynia and the transition from acute to chronic pain.41 There are some studies that suggest that use of intraoperative ketamine, a potent NMDA antagonist, can reduces the development of chronic postsurgical pain 3 and 6 months after surgery.42 At the present time, however, there is only limited evidence documenting that any perioperative agent can consistently reduce the risk of chronic pain after surgery.
Methadone is a NMDA antagonist like ketamine9,10 and has also been documented to reduce the intensity of post- operative pain.Therefore, it is possible that a single dose of intraoperative methadone may have a preventive analgesic effect and decrease the risk of the development of chronic postsurgical pain. Komen et al.29 observed that ambula- tory patients given 0.15mg/kg of methadone at anesthetic induction had significantly less pain at rest and required fewer oral opioids for the first 30 days after surgery com- pared to those given shorter-acting intraoperative opioids. Longer-term follow-up for the development of chronic postsurgical pain was not conducted in this study or in most other investigations examining perioperative methadone use (1-yr follow-up is currently being conducted in two investigations in cardiac and spine patients).18,26
What Is the risk of postoperative respiratory Depression Associated with methadone, When Compared with Shorter-acting Opioids?
Clinicians may have concerns that the long half-life of methadone may contribute to prolonged sedation and respiratory depression.Clinical trials have not supported this belief. However, continuous pulse oximetry and respiratory rate monitoring was not used in any of the investigations for the first 24 to 72 postoperative h to compare the inci- dences of adverse respiratory events between methadone and control groups, and studies were not adequately pow- ered to assess this important outcome measure.A retrospec- tive analysis of a large cohort of patients undergoing major spine surgery reported that 37% of patients given meth- adone experienced postoperative respiratory depression.33 However, this cohort was not compared to a control group not administered methadone. Furthermore, all patients were given additional opioids intraoperatively and likely required a significant amount of intravenous hydromorphone after major, multilevel spine surgery (data not reported).
If naloxone is required in the PACU for methadone- induced respiratory depression, an infusion should be considered. The half-life of naloxone (approximately 90min) is considerably shorter than that of methadone (35 h with a dose of 20 mg). Recurrent respiratory depres- sion has been reported in a patient given a single dose of naloxone after cardiac surgery.32
Is there a role for methadone in enhanced recovery after Surgery protocols?
An important component of most enhanced recovery after surgery protocols is a reduction in the use of intra- and postoperative opioids, primarily to minimize the adverse effects of these medications on respiratory and bowel func- tion. Only one study has specifically addressed the effect of methadone on postoperative bowel function.18 The reduction in need for postoperative opioids associated with methadone use may provide a beneficial effect on bowel
motility. In contrast, the prolonged elimination phase of methadone may adversely affect recovery of bowel motil- ity. Further research is needed to determine the effect of methadone on bowel function, patient-perceived quality of recovery, hospital length of stay, and outcomes after dis- charge in enhanced recovery after surgery protocols.At the present time, only one published investigation has exam- ined the use of methadone as part of an enhanced recovery after surgery pathway (in patients undergoing spinal fusion for idiopathic scoliosis).43
Many of the clinical trials examined the effect of methadone on postoperative analgesia in the absence of other opioid-sparing agents to avoid the potential con- founding effects of these other agents. In the investiga- tion by Singhal et al.,31 the addition of methadone to a standardized multimodal approach to pain management resulted in lower pain scares, decreased analgesic require- ments, and a shorter hospital length of stay. Furthermore, studies that included the addition of other opioid-spar- ing agents in both the methadone and control treatment groups reported that pain scores and analgesic use were less in the methadone groups.18,23 Additional investiga- tions are needed to define the role of methadone as part of a multimodal treatment strategy for postoperative pain and enhanced recovery.
Caution may be required when methadone is com- bined with other agents as part of an enhanced recovery after surgery protocol.The administration of opioids with gabapentinoids has been reported to increase the risk of postoperative respiratory depression.44 In contrast, there may be a beneficial effect of the combination of metha- done and ketamine in the perioperative period. A syner- gic antinociceptive effect of ketamine and methadone has been demonstrated in experimental neuropathy,45 and the use of these agents together by patient-controlled analge- sic administration has been shown to significantly decrease opioid consumption46
Conclusions
Methadone is a long-acting opioid with a unique phar- macokinetic profile. It has additional central nervous sys- tem effects (NMDA receptor antagonism and inhibition of serotonin and norepinephrine uptake)9–14 that may enhance recovery by attenuating the development of hyperalge- sia and tolerance and improve mood state. Randomized clinical trials in patients undergoing a variety of surgical procedures have documented that the use of methadone in the operating room is associated with significant reduc- tions in postoperative analgesic requirements, compared to patients administered shorter-acting intraoperative opi- oids. In addition, most studies also demonstrated that pain scores were significantly lower in patients given methadone. The risk of opioid-related side effects was not increased in the methadone groups in any of the randomized clinical investigations.
For procedures associated with higher levels of postopera- tive pain (major spine or open abdominal or thoracic), a dose of 20 mg at induction of anesthesia has been demonstrated to provide long-lasting analgesia with a minimal risk of post- operative respiratory depression. Smaller doses (10 to 15 mg) have been administered in the elderly or those with limited physiologic reserve due to existent comorbidities.4 The care- ful titration of additional methadone in the PACU (3 to 5 mg with at least 20min between doses) can further prolong the duration of postoperative analgesia. For procedures associated with moderate levels of postoperative pain (laparoscopic pro- cedures), a dose of 10 mg before surgical incision will provide sufficient postoperative analgesia in most patients.
The majority of studies have used a single dose of meth- adone at induction of anesthesia and avoided the use of other intraoperative opioids. The investigation by Porter et al.16 documented that the administration of methadone before surgery was more effective in reducing postoperative analgesic requirements than a dose given at surgical closure. Furthermore, the peak respiratory depressant effect of meth- adone occurs approximately 8 to 10min after administra- tion.4 When an appropriate dose is given at induction of anesthesia, the peak respiratory depressant effect occurs at a time when the airway is controlled, and the duration of sur- gery will allow sufficient time for spontaneous recovery of ventilation. Due to the long half-life of methadone, there are limited data to suggest that repeat dosing is required in the operating room. If opioid-induced respiratory depression is suspected after the administration of methadone, a naloxone infusion may be required, and careful respiratory monitoring is indicated for the first 24 to 48h.
The reasons why methadone is not more commonly administered to surgical patients (outside of complex spine surgery) are uncertain but may be related to misconcep- tions about pharmacokinetics and duration of action of the agent, concerns about prolonged respiratory depres- sion after its administration, or limited published litera- ture supporting its use in the perioperative setting. At the present time, the majority of investigations have been rela- tively small in size and should be considered “pilot studies.” Further, larger-scale, randomized trials are required to more clearly define the efficacy and safety of methadone use in the perioperative period. Data from such trials are needed before the routine use of methadone in surgical patients can be recommended. Optimal dosing regimens in various surgical procedures, as well as appropriate use in high-risk patient populations, has yet to be determined. In addition, the risk of postoperative respiratory depression, when com- pared to shorter-acting opioids, has not been definitively established. Finally, studies to determine the potential ben- eficial effects of a dose of intraoperative methadone on quality of recovery variables, bowel function, and hospital length of stay in enhanced recovery after surgery protocols, as well as the development of chronic postsurgical pain, are required.
Research Support
Supported by the Department of Anesthesiology, NorthShore University HealthSystem, Evanston, Illinois.
Competing Interests
Dr. Murphy has served on the Advisory Board and as a speaker for Merck (Kenilworth, New Jersey). Dr. Szokol declares no competing interests.
Correspondence
Address correspondence to Dr. Murphy: NorthShore University HealthSystem, 2650 Ridge Avenue, Evanston, Illinois 60201. dgmurphy2@yahoo.com.This article may be accessed for personal use at no charge through the Journal Web site, www.anesthesiology.org.
References
Fletcher D,Fermanian C,MardayeA,Aegerter P;Pain and Regional Anesthesia Committee of the French Anesthesia and Intensive Care Society (SFAR): A patient-based national survey on postoperative pain management in France reveals significant achievements and persistent challenges. Pain 2008; 137:441–51
Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W: Pain intensity on the first day after surgery:A prospective cohort study com- paring 179 surgical procedures.Anesthesiology 2013; 118:934–44
Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M: Enhanced recovery program in colorectal surgery:A meta-analysis of randomized controlled tri- als.World J Surg 2014; 38:1531–41
Kharasch ED: Intraoperative methadone: Rediscovery, reappraisal, and reinvigoration? Anesth Analg 2011; 112:13–6
Mattick RP, Breen C, Kimber J, Davoli M: Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; 8:CD002209
Gourlay GK,Wilson PR,Glynn CJ:Pharmacodynamics and pharmacokinetics of methadone during the periop- erative period. Anesthesiology 1982; 57:458–67
Gourlay GK,Willis RJ,Wilson PR: Postoperative pain control with methadone: Influence of supplementary methadone doses and blood concentration–response relationships. Anesthesiology 1984; 61:19–26
Inturrisi CE, Colburn WA, Kaiko RF, Houde RW, Foley KM: Pharmacokinetics and pharmacodynam- ics of methadone in patients with chronic pain. Clin Pharmacol Ther 1987; 41:392–401
9. Davis AM, Inturrisi CE: d-Methadone blocks mor- phine tolerance and N-methyl-d-aspartate–induced hyperalgesia.J Pharmacol ExpTher 1999;289:1048–53
10. Sotgiu ML,Valente M, Storchi R, Caramenti G, Biella GE: Cooperative N-methyl-d-aspartate (NMDA) receptor antagonism and μ-opioid receptor agonism mediate the methadone inhibition of the spinal neu- ron pain-related hyperactivity in a rat model of neuro- pathic pain. Pharmacol Res 2009; 60:284–90
11. Zhao YL, Chen SR, Chen H, Pan HL: Chronic opi- oid potentiates presynaptic but impairs postsynaptic N-methyl-d-aspartic acid receptor activity in spinal cords: Implications for opioid hyperalgesia and toler- ance. J Biol Chem 2012; 287:25073–85
12. Richebé P, Julien M, Brulotte V: Potential strategies for preventing chronic postoperative pain: A practical approach: Continuing Professional Development. Can J Anaesth 2015; 62:1329–41
13. CoddEE,ShankRP,SchupskyJJ,RaffaRB:Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: Structural determinants and role in ant- inociception. J Pharmacol Exp Ther 1995; 274:1263–70
14. Rojas-Corrales MO, Berrocoso E, Gibert-Rahola J, Micó JA: Antidepressant-like effects of tramadol and other central analgesics with activity on monoamines reuptake, in helpless rats. Life Sci 2002; 72:143–52
15. Gourlay GK, Willis RJ, Lamberty J: A double-blind comparison of the efficacy of methadone and mor- phine in postoperative pain control. Anesthesiology 1986; 64:322–7
16. Porter EJ, McQuay HJ, Bullingham RE, Weir L, Allen MC, Moore RA: Comparison of effects of intraoper- ative and postoperative methadone:Acute tolerance to the postoperative dose? Br J Anaesth 1983; 55:325–32
17. Gottschalk A, Durieux ME, Nemergut EC: Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg 2011; 112:218–23
18. Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear TD, Deshur MA,Vender JS, Benson J, Newmark RL: Clinical effectiveness and safety of intraoperative methadone in patients undergoing posterior spinal fusion surgery: A randomized, double-blinded, con- trolled trial. Anesthesiology 2017; 126:822–33
19. Sharma A, Tallchief D, Blood J, Kim T, London A, Kharasch ED: Perioperative pharmacokinetics of methadone in adolescents. Anesthesiology 2011; 115:1153–61
20. Stemland CJ, Witte J, Colquhoun DA, Durieux ME, Langman LJ, Balireddy R, Thammishetti S, Abel MF, Anderson BJ:The pharmacokinetics of methadone in adolescents undergoing posterior spinal fusion. Paediatr Anaesth 2013; 23:51–7
21. Chui PT, Gin T: A double-blind randomised trial comparing postoperative analgesia after perioperative
22. Loading doses of methadone or morphine. Anaesth Intensive Care 1992; 20:46–51
Richlin DM, Reuben SS: Postoperative pain control with methadone following lower abdominal surgery. J Clin Anesth 1991; 3:112–6
23. Russell T, Mitchell C, Paech MJ, Pavy T: Efficacy and safety of intraoperative intravenous methadone during general anaesthesia for caesarean delivery:A retrospective case-control study. Int J Obstet Anesth 2013; 22:47–51
24. Udelsmann A, Maciel FG, Servian DC, Reis E, de Azevedo TM, Melo Mde S: Methadone and mor- phine during anesthesia induction for cardiac surgery: Repercussion in postoperative analgesia and prevalence of nausea and vomiting. Rev Bras Anestesiol 2011; 61:695–701
25. Carvalho AC, Sebold FJG, Calegari PMG, Oliveira BH, Schuelter-Trevisol F: [Comparison of postoperative analgesia with methadone versus morphine in cardiac surgery]. Rev Bras Anestesiol 2018; 68:122–7
26. Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Marymont JH,ShearT,Parikh KN,Patel SS,Gupta DK: Intraoperative methadone for the prevention of post- operative pain: A randomized, double-blinded clinical trial in cardiac surgical patients.Anesthesiology 2015; 122:1112–22
27. Simoni RF, Cangiani LM, Pereira AM, Abreu MP, Cangiani LH, Zemi G: [Efficacy of intraoperative methadone and clonidine in pain control in the imme- diate postoperative period after the use of remifent- anil]. Rev Bras Anestesiol 2009; 59:421–30
28. Rivosecchi RM, Rice MJ, Smithburger PL, Buckley MS, Coons JC, Kane-Gill SL: An evidence based sys- tematic review of remifentanil associated opioid-in- duced hyperalgesia. Expert Opin Drug Saf 2014; 13:587–603
29. Komen H, Brunt LM, Deych E, Blood J, Kharasch ED: Intraoperative methadone in same-day ambulatory surgery: A randomized, double-blinded, dose-finding pilot study. Anesth Analg 2019; 128:802–10
30. Berde CB, Beyer JE, Bournaki MC, Levin CR, Sethna NF: Comparison of morphine and methadone for pre- vention of postoperative pain in 3- to 7-year-old chil- dren. J Pediatr 1991; 119:136–41
31. Singhal NR, Jones J, Semenova J, Williamson A, McCollum K,Tong D, Jerman J, Notrica DM, Nguyen H: Multimodal anesthesia with the addition of meth- adone is superior to epidural analgesia:A retrospective comparison of intraoperative anesthetic techniques and pain management for 124 pediatric patients undergo- ing the Nuss procedure. J Pediatr Surg 2016; 51:612–6
32. Norris JV, Don HF: Prolonged depression of respiratory rate following methadone analgesia. Anesthesiology 1976; 45:361–2
Dunn LK,Yerra
33. Dunn LK,Yerra S, Fang S, Hanak MF, Leibowitz MK, Alpert SB, Tsang S, Durieux ME, Nemergut EC,
Naik BI: Safety profile of intraoperative methadone for analgesia after major spine surgery: An observa- tional study of 1,478 patients. J Opioid Manag 2018; 14:83–7
34. Baldo BA: Opioid analgesic drugs and serotonin toxic- ity (syndrome): Mechanisms, animal models, and links to clinical effects. Arch Toxicol 2018; 92:2457–73
35. Kharasch ED,Regina KJ,Blood J,Friedel C:Methadone pharmacogenetics: CYP2B6 polymorphisms deter- mine plasma concentrations, clearance, and metabo- lism. Anesthesiology 2015; 123:1142–53
36. Cozowicz C, Chung F, Doufas AG, Nagappa M, Memtsoudis SG: Opioids for acute pain management in patients with obstructive sleep apnea: A systematic review. Anesth Analg 2018; 127:988–1001
37. Alinejad S, Kazemi T, Zamani N, Hoffman RS, Mehrpour O:A systematic review of the cardiotoxicity of methadone. EXCLI J 2015; 14:577–600
38. Johnston J, Pal S, Nagele P: Perioperative torsade de pointes:A systematic review of published case reports. Anesth Analg 2013; 117:559–64
39. Nagele P, Pal S, Brown F, Blood J, Miller JP, Johnston J: Postoperative Q. T interval prolongation in patients undergoing noncardiac surgery under general anesthe- sia. Anesthesiology 2012; 117:321–8
40. Kehlet H, Jensen TS, Woolf CJ: Persistent postsurgi- cal pain: Risk factors and prevention. Lancet 2006; 367:1618–25
41. Moyse DW, Kaye AD, Diaz JH, Qadri MY, Lindsay D, Pyati S: Perioperative ketamine administration for tho- racotomy pain. Pain Physician 2017; 20:173–84
42. McNicol ED, Schumann R, Haroutounian S: A sys- tematic review and meta-analysis of ketamine for the prevention of persistent post-surgical pain. Acta Anaesthesiol Scand 2014; 58:1199–213
43. Muhly WT, Sankar WN, Ryan K, Norton A, Maxwell LG, DiMaggio T, Farrell S, Hughes R, Gornitzky A, Keren R, McCloskey JJ, Flynn JM: Rapid recovery pathway after spinal fusion for idiopathic scoliosis. Pediatrics 2016; 137:e20151568
44. Cavalcante AN, Sprung J, Schroeder DR, Weingarten TN: Multimodal analgesic therapy with gabapen- tin and its association with postoperative respiratory depression. Anesth Analg 2017; 125:141–6
45. Pelissier T, Laurido C, Kramer V, Hernández A, Paeile C:Antinociceptive interactions of ketamine with mor- phine or methadone in mononeuropathic rats. Eur J Pharmacol 2003; 477:23–8
46. Pacreu S, Fernández Candil J, Moltó L, Carazo J, Fernández Galinski S:The perioperative combination of methadone and ketamine reduces post-operative opioid usage compared with methadone alone. Acta Anaesthesiol Scand 2012; 56:1250–6
